Pharmaceuticals Solutions
With more than a century of experience, dedicated production lines and specialized R&D and regulatory teams, ADM offers its customers innovative global solutions tailored to the specific challenges of the pharmaceutical industry.


Expert Solutions to Pharmaceutical Challenges
ADM offers an extensive range of ingredients to meet your pharmaceutical needs, all manufactured under strict quality and regulatory conditions to ensure compliance.
Our products can be integrated in a wide range of pharmaceutical applications.
We are committed to providing only the purest and highest quality products for your end solutions.
Parenteral Nutrition & Injectables
With an increased demand for nutritional support and drugs, highly purified oils that meet regulation standards have become essential in the formulation of parenteral nutrition as an active pharmaceutical ingredient (API) or excipient, and for injectables as excipients.
Our highly purified olive oil is fully compliant with the most demanding regulations and can be used as an excipient or as an API in parenteral nutrition. ADM' refining process mitigates variability of oils to guarantee batch to batch consistency.

Highly purified and fully compliant with the most demanding regulations, soybean oil is ideal to use as an excipient or as an API in parenteral nutrition. It is manufactured under cGMP according to the ICH-Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients.

Highly purified olive oil can be used as an excipient for the formulation of poorly soluble drugs. Our multi-step purification process ensures high quality and consistency of the final product for intravenous applications.
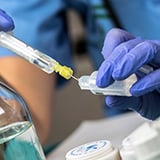
Highly purified Soybean oil can be used as an excipient for the formulation of poorly soluble drugs. With our refining process, we can mitigate variability to guarantee batch-to-batch consistency.

Highly purified Sesame oils can be used as an excipient in the formulation of oral pharmaceutical drugs such as syrups and soft gels.
Oral & Topical
Available from a variety of different vegetable sources, ADM pharmaceutical ingredients can be used as excipients in topical and oral formulations. ADM's refined and hydrogenated oils, carbohydrate ingredients and more, are appropriate for use in soft gels, capsules, creams, lotions and oral formulations such as syrups and medical nutrition.

Highly purified olive oil can be used as an excipient in the formulation of oral pharmaceutical products such as soft gels and capsules.

Highly purified Soybean oil can be used as an excipient in the formulation of oral pharmaceutical drugs such as syrups and capsules.

Highly purified Sesame oils can be used as an excipient in the formulation of oral pharmaceutical drugs such as syrups and soft gels.

Novatol™ is a naturally derived, fat-soluble vitamin E that offers a range of benefits. It’s twice as bioavailable as synthetic Vitamin E and is compliant with European pharmacopoeia monographs. Among its many potential features, Vitamin E may display antioxidant effects, support physiological, cardiometabolic and neurological function, as well as support the immune system.

Our portfolio of plant-based carbohydrates includes dextrose monohydrates, crystallin fructose and maize starch. It offers functionality, taste, and caloric value, and facilitates the delivery of drugs, aiding in the production of tablets, capsules, liquids, and more. Monosaccharides offer a sweet flavor, and our native starch can work as a thickening agent for liquid products.

Propylene Glycol (PG USP/EP) is a pharmacopeia-specifications-grade excipient of monopropylene glycol (PG or MPG) with a specified purity greater than 99.5%. Propylène glycol USP/EP is tested for compliance with the current United States Pharmacopeia (USP) and can be used as a pharmaceutical ingredient in the formulation of oral pharmaceutical solutions.

Highly purified olive oil can be used in the formulation of topical drug as an excipient. Our multi-step purification process ensures high quality and consistency of the final topical solution.

Highly purified, fully compliant soybean oil can be used in the formulation of topical drugs as an excipient. With our refining process, we can mitigate variability to guarantee batch-to-batch consistency.

Following a multi-step purification process, highly purified sesame oil can be used as an excipient in the formulation of lipophilic drugs for topical application for human and veterinary solutions.

Novatol™ is a naturally derived, fat-soluble vitamin E that offers a range of benefits. It’s twice as bioavailable as synthetic Vitamin E and is compliant with European pharmacopoeia monographs. Among its many potential features, Vitamin E may display antioxidant effects, support physiological, cardiometabolic and neurological function, as well as support the immune system.

Our plant-based carbohydrate solutions (Dextrose Monohydrate 200 & 300, Crystallin Fructose C&M, Maize Starch) provide functionality such as humectant properties and texture to address formulation needs and help in the production of topical drugs. Native starch can work as a thickening agent for liquid formulations.

Propylene Glycol (PG USP/EP) is a pharmacopeia-specifications-grade excipient of monopropylene glycol (PG or MPG) with a specified purity greater than 99.5%. Propylene glycol USP/EP is tested for compliance with the current United States Pharmacopeia (USP) and can be used as a pharmaceutical ingredient in the formulation of topical pharmaceutical solutions.
For general inquiries, questions, or more information, please reach out to us. We are here to help.

Hi! We're Performing Maintenance.
ERROR CODE: 503
Here are some helpful links
Hello
Need some more information? Complete this contact form and you will be contacted in the next 5 business days.
This content is based on United States laws and regulations applicable on the day of publication of this content. We point out that customers are required to ensure that any labeling and claims made by customers for their finished products must be based on the regulatory requirements and scientific standards of the country in which the final products are offered for sale. Not all products are available in all regions.